In the same section
- Home
- Units
- Light, nanomaterials, nanotechnologies (L2n - CNRS-UMR 7076)
- Research topics
- Biophotonics and nanosensors
Nanobiophotonics and Nanosensors
Topic coordinator: Rodolphe Jaffiol
Summary
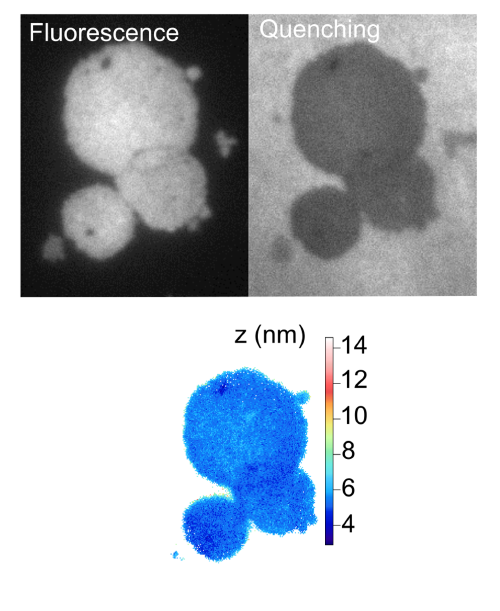
Since 2006, we have been developing techniques based on fluorescence spectroscopy and imaging to study living cells. Our ambition is to propose different tools, allowing quantitative analysis at single cell level. Single molecule spectroscopy such as FCS (Fluorescence Correlation Spectroscopy) in diffracted limited and in sub-diffracted volume is employed to probe diffusion process at the nanoscale on cell membrane. Fluorescence nanoscopy, such as variable-angle Total Internal Reflection Fluorescence (vaTIRF) and Non-radiative Excitation Fluorescence (NEF), are used to investigate membrane/substrate interactions and cell adhesion.Keywords: Single molecule, Fluorescence Correlation Spectroscopy (FCS), fluorescence nano-imaging, super-resolution, Förster Resonant Energy Transfer (FRET), Total Internal Reflection Fluorescence (TIRF), Cell adhesion and migration, membrane fluidity and organization.
Research highlights by keywords
- Biosensors
-
Accordion content
- Cell adhesion
- Cell nano-imaging
-
CCell
- Electromagnetic modeling of biological structures
- Nano-toxicity and environmental safety
-
Saisissez ici le contenu ici
Current research projects and related applications
Non-exhaustive list in alphabetic order- Biosensors (S. Zeng, E. Ionescu, Q. Jiang, J-L. Bijeon)
- Cell adhesion and migration (R. Jaffiol, C. Vézy)
- Cell nano-imaging (R. Jaffiol, C. Vézy)
- Electromagnetic modeling of biological structures (D. Macias, A. Vial)
- Nano-toxicity and environmental safety (E. Ionescu, J. Proust)
Date of update 04 mai 2023